Background : Myelofibrosis (MF) is a rare myeloproliferative disorder associated with significant morbidity and mortality. The hallmarks of MF pathobiology include aberrant myeloproliferation, cytokine-induced inflammation, bone marrow reticulin fibrosis, extramedullary hematopoiesis, cytopenias, and constitutional symptoms. Hydroxyurea was the most commonly used drug in patients with MF prior to the approval of ruxolitinib (RUX), a first-in-class Janus kinase 1/2 inhibitor (JAKi) that is widely approved for symptomatic patients with MF. In addition to RUX, the JAKis fedratinib and, more recently, pacritinib have also been approved by the US Food and Drug Administration. Real-world treatment patterns and impact of currently available JAKis on patients with MF are not well understood. Several novel agents are in advanced development and will further reshape the treatment landscape of MF. A better understanding of current treatment patterns will assist in positioning novel treatments within the MF therapeutic landscape.
Methods : The METER study (NCT05444972) is an ongoing multicountry noninterventional retrospective chart review assessing treatment patterns, effectiveness, and healthcare resource utilization (HCRU) in patients diagnosed with MF. Data from patients aged ≥18 years with primary or secondary MF treated on or after the local first date of RUX approval until December 31 , 2021 were assessed. Patients who received treatment for MF in a clinical trial were excluded. The primary objective is to describe real-world MF treatment patterns, including patient characteristics, time from MF diagnosis to first-line (1L) therapy, choice, duration and reason for change/discontinuation of initial and subsequent treatments for MF, and treatment procedures. Secondary objectives include assessments of days hospitalized as part of HCRU.
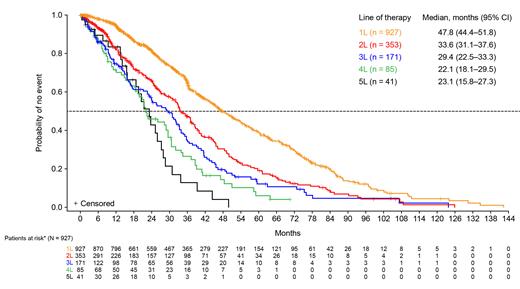
Results : As of June 1, 2023, 928 patient charts were included, met eligibility criteria, and had initial treatment information available. Most patients were male (54%) and White (65%), and the median (range) age for patients ≤89 years of age was 66 (58-73) years; the most common geographic locations were Europe (53%), Latin America (23%), and North America (13%). Of patients, 66% (614/928) had primary MF, and 10% (97/928) were transfusion-dependent. Of patients tested for high molecular risk mutations, 50% (81/162) were positive. Of patients with available bone marrow fibrosis data, 82% (554/680) had grade ≥2 bone marrow fibrosis at diagnosis. The average interval from MF diagnosis to start of initial treatment (index date) was approximately 8.5 months. RUX was the most commonly used 1L therapy (47%; 437/928) followed by hydroxyurea (41%; 380/928). RUX was also the most common therapy used in second-line (2L), third-line, fourth-line, and fifth-line treatment for 62% (217/353), 63% (107/171), 66% (56/85), and 59% (24/41) of patients, respectively. Mean (SD) time from index date to procedural intervention was 556 (519) days; the most common procedure was stem cell transplant (n = 71) followed by splenectomy (n = 20). Of the 927 patients who received 1L therapy, 98% remained on 1L therapy through Week 24, and 66% did not initiate 2L therapy until Week 156. Median (95% CI) duration of 1L therapy was 48 (45-52) months (Figure). Median (95% CI) survival from the start of 1L therapy was 79 (71-NR) months. The estimated survival rate (95% CI) was 76% (73-79) at Week 156. Median (95% CI) overall survival was 120 (93-147) months. Median (Q1-Q3) total number of days hospitalized after the index date was 2 (0-19), and median (Q1, Q3) total number of days in the intensive care unit after the index date was 0 (0, 0). Analyses will be updated for presentation.
Conclusions : In patients with MF, RUX was the most commonly used agent in all lines of therapy. The greatest reduction in duration of MF treatment occurred from 1L to 2L. Most patients remained on 1L therapy through Week 24 and did not initiate 2L therapy until Week 156. Median survival time from start of 1L therapy to death was 79 months (approximately 6 years). There was a high degree of advanced bone marrow fibrosis as well as transfusion dependence among this real-world patient population. Together, these results highlight the importance of optimizing 1L therapy.
Disclosures
Gupta:Pfizer: Consultancy, Other: Participation on a Data Safety Monitoring Board or Advisory Board; Constellation Biopharma: Consultancy; GSK Pharma: Consultancy, Honoraria, Other: Support for attending meetings and/or travel; CTI Biopharma: Consultancy, Other: Participation on a Data Safety Monitoring Board or Advisory Board; Roche: Other: Participation on a Data Safety Monitoring Board or Advisory Board; AbbVie: Consultancy, Honoraria, Other: Participation on a Data Safety Monitoring Board or Advisory Board; Sumitomo Pharma: Consultancy; BMS Celgene: Consultancy, Other: Participation on a Data Safety Monitoring Board or Advisory Board; Novartis: Consultancy; Sierra Oncology: Consultancy, Other: Participation on a Data Safety Monitoring Board or Advisory Board. Lampon:AbbVie: Other: investigator on AbbVie-sponsored clinical trials. Hou:AbbVie: Consultancy, Honoraria, Other: investigator on AbbVie-sponsored clinical trials; travel, Research Funding; BMS: Consultancy, Honoraria, Other: travel, Research Funding; Celgene: Consultancy, Honoraria, Other: travel, Research Funding; Kirin: Consultancy, Honoraria, Other: travel, Research Funding; PharmaEssential: Consultancy, Honoraria, Other: travel, Research Funding; Astellas: Consultancy, Other: travel; BeiGene: Consultancy, Honoraria, Other: travel; Chugai: Consultancy, Honoraria, Other: travel; CSL Behring: Consultancy, Honoraria, Other: travel; Daiichi Sankyo: Consultancy, Honoraria, Other: travel; IQVIA: Consultancy, Honoraria, Other: travel; Johnson & Johnson: Consultancy, Honoraria, Other: travel; Lotus: Consultancy, Honoraria, Other: travel; Merck Sharp & Dohme: Consultancy, Honoraria, Other: travel; Novartis: Consultancy, Honoraria, Other: travel; Ono: Consultancy, Honoraria, Other: travel; Panco healthcare Co: Consultancy, Honoraria, Other: travel; Pfizer: Consultancy, Honoraria, Other: travel; Roche: Consultancy, Honoraria, Other: travel; Synmosa: Consultancy, Honoraria, Other: travel; Takeda: Consultancy, Honoraria, Other: travel; TSH Biopharm: Consultancy, Honoraria, Other: travel; TTY Biopharm Company: Consultancy, Honoraria, Other: travel; Zuellig Pharma: Consultancy, Honoraria, Other: travel. Helbig:AbbVie: Other: investigator on AbbVie-sponsored clinical trials; Gilead: Honoraria; Novartis: Speakers Bureau; GSK: Honoraria; Swixx: Honoraria. Symeonidis:Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Haznedaroglu:AbbVie: Other: investigator on AbbVie-sponsored clinical trials. Galvez:Boehringer Ingelheim: Honoraria; AbbVie: Other: investigator on AbbVie-sponsored clinical trials; Sanofi: Research Funding. Tatsch:AbbVie: Current Employment, Current holder of stock options in a privately-held company. Chopra:AbbVie: Current Employment, Current holder of stock options in a privately-held company. Zhang:AbbVie: Other: investigator on AbbVie-sponsored clinical trials. Vizkelety:AbbVie: Current Employment, Current holder of stock options in a privately-held company. Ross:Keros: Consultancy, Other: investigator; Takeda: Membership on an entity's Board of Directors or advisory committees; Menarini: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: lectures, investigator, Research Funding; AbbVie: Other: investigator on AbbVie-sponsored clinical trials; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Other: lectures, investigator, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal